Do You Need an ERP System for Medical Devices and Implants?
The medical device sector plays a critical role in the healthcare industry, but evolving customer demands, supply-chain disruptions, and inventory mismanagement have become major hurdles for the industry.
For instance, McKinsey estimates medical device product warranty or recalls alone cost the medical device industry at least $5 billion annually. A single manufacturer facing a product recall can expect to part with up to $600 million in addition to facing expensive lawsuits and other costs related to product recalls. McKinsey also documented a 13% drop in a company’s stock price anytime a brand is attached to quality issues.

Add credibility costs and the potential confidence drops that result in lost revenue as consumers and investors look elsewhere, and we begin to see the problem. Manufacturers must ensure product quality and availability despite these ever-growing challenges.
The problem is clear, medical devices companies need to find an improved way to manage medical device sales but tracking individual medical devices isn’t easy and comes with its own challenges.
That said, one of the most popular and effective ways to manage medical device inventory is to use an Enterprise Resource Planning (ERP) system. The software is designed to facilitate hassle-free data sharing and access functionality by breaking down silos and supporting production, procurement, and warehousing operations. This article will discuss the importance of obtaining an ERP system and how it helps in compliance, automation, order fulfillment, and product tracking.
What is an ERP System?
ERP is a blended “Enterprise Resource Management” process through which an organization gathers and organizes business data using an integrated software suite. In other words, ERP is an intuitive piece of software that helps streamline various business functions including billing, scheduling, inventory management, and accounting.
ERP simplifies interdepartmental company operations and facilitates the improved handling of all business resources and assets Some benefits of ERP software include:
- Ease of Use: Most ERP systems have an easy-to-use dashboard with a user-friendly interface that allows employees to integrate it into their workflows without extensive training.
- Data Centralization: The system consolidates company data in a single, resilient database (ideally cloud-based). This model is called the Single Source of Truth (SSOT) model and is widely adopted in modern enterprises to avoid data duplication and errors. ERP systems also make data easily accessible to designated employees.
- Regulatory Optimization: Most ERP systems will support compliance, albeit to varying extents. The right software will ensure you deploy all the necessary protocols to ensure your medical device manufacturing process adheres to the strictest regulatory standards.
- Better Harmonization: ERP systems facilitate real-time data and device visibility resulting in improved decision making across the organization.

Uses Cases of an ERP System for Medical Devices and Implants
Regulatory bodies such as the FDA demand strict quality assurance and challenging timetables from medical device companies. This means medical device companies lacking ERP in a rapidly growing business environment cannot compete favorably with those that do. Research by Software Advice revealed two-thirds of manufacturers don’t use ERP systems, while 44% use different techniques.
But for those that do use it, ERP provides an innovative way to confront current industry challenges that most medical device manufacturers struggle with. Let’s take a look a closer look at how ERP systems are used to better manage medical device operations:
1. Compliance
The critical nature of the use for medical devices means regulatory bodies have placed a long list of regulations and standards for manufacturers to adhere to. The slightest compliance failure of any of the rules is a costly affair. Medical device manufacturers use ERP systems to stay compliant with operational regulations and stay within availability limits to meet customer demands.
ERP systems streamline the process by providing users with the functionality they need to adhere to regulations throughout a device’s lifecycle from manufacturing to distribution.
2. Implementation of Automation
There’s no room for error for medical device manufacturers and strict precision is necessary. Deploying a medical device ERP helps to eliminate risks of human error throughout the medical device lifecycle until billing.
ERP systems achieve this by automating a wide variety of processes, including journal entries, daily accounting tasks, calculating inventory levels, managing billing, and more.
3. Fulfilling Orders
From inquiry to product delivery, the sales process should be so efficient that there’s no room for low inventory returns or stock-outs. A well-implemented ERP system acts as the launching pad for all information about procurement, shipping, purchases, and inventory. The system facilitates order planning as information is delivered on time, meaning everyone in the pipeline has enough time to perform their roles.
4. Visibility across Industry
The medical device industry resembles a fast-moving massive machine with thousands of small moving parts that must be well-oiled to move efficiently. Medical device ERP facilitates visibility across the enterprise.
5. Real-time Product Tracking
ERP software can be customized to automate product tracking for individual devices by serial and lot numbers. This functionality facilitates single-level to multi-level sampling besides enabling 100% product inspection. The system offers features like batch and serial number tracking, thereby simplifying the process of quality control operations.
Must-have Features in a Medical Device ERP System
The medical device industry is among the fastest sectors anywhere in the world today thanks to easier access to healthcare. This also means that the market for ERP systems built specifically for medical devices is also growing. That said, not all ERP systems are built the same and only the right ERP system will guarantee a competitive edge in our highly regulated industry.
To help you choose the best medical ERP system available, we’ve compiled the following list of the most important features in a medical device ERP system:
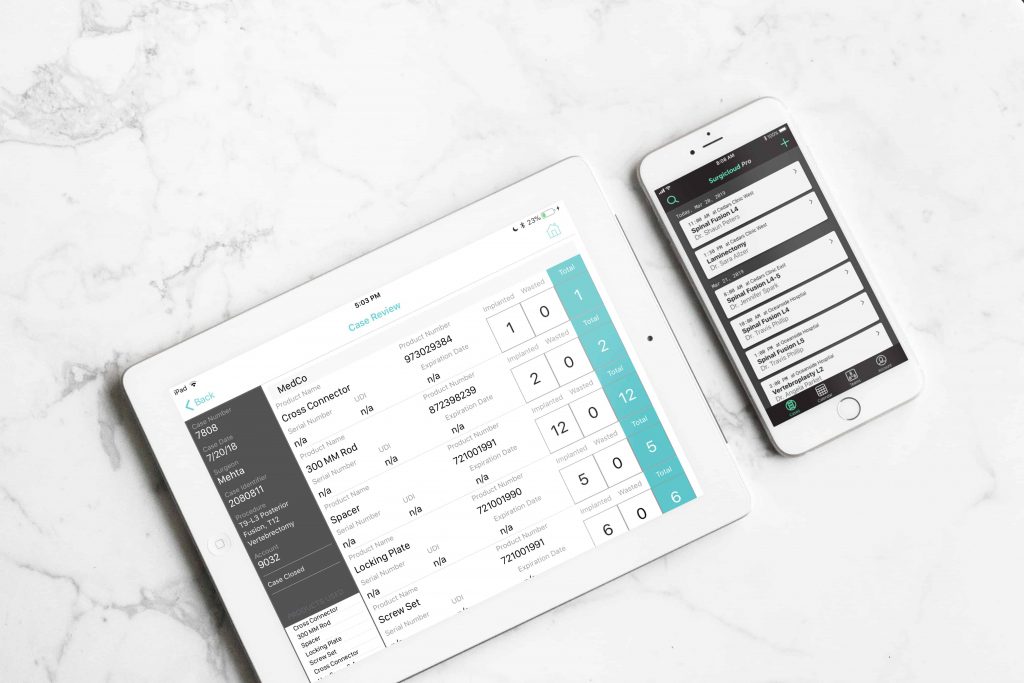
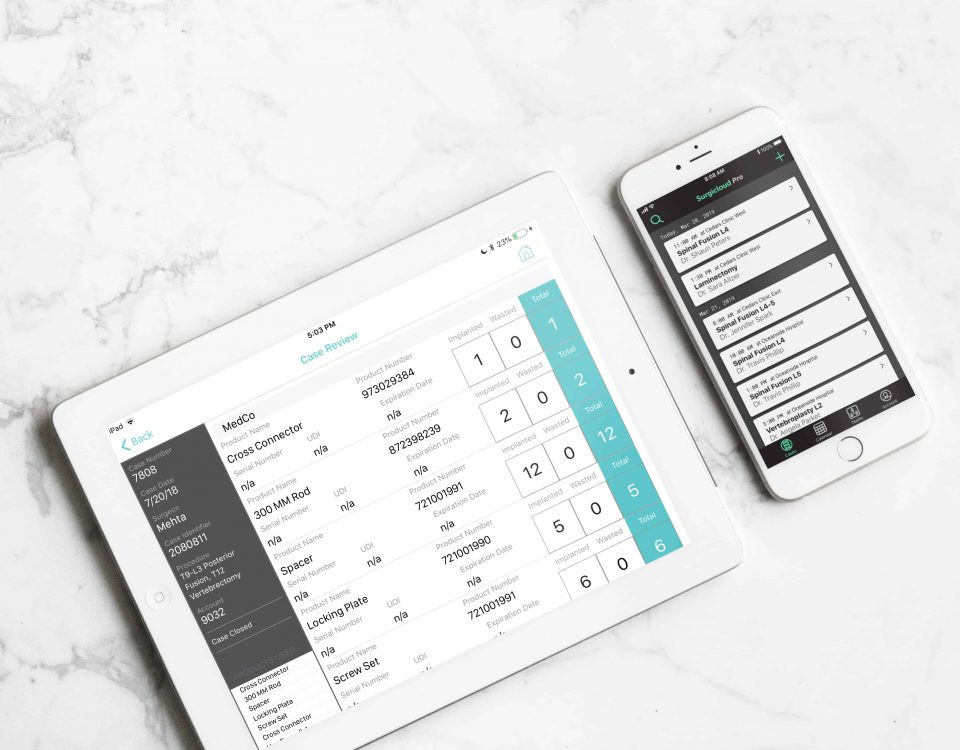
1. Live Intraoperative Implant Tracking
An effective ERP solution should facilitate serial control from a device’s manufacturing to surgery. However, tracking implants post-manufacturing or post-surgery is bad practice and opens up the company to numerous challenges.
The ideal medical ERP system will therefore support live intraoperative implant tracking. This will enable users to log medical device data from the assembly line to the OR in real-time. This significantly reduces the time and resources spent preparing documents for the billing cycle.
2. Case-by-case/Team Management
The ideal ERP system will have the functionality to sort and filter data on the basis of cases and teams. Team features facilitate collaboration and improve interdepartmental productivity. It also improves visibility, ensuring nothing goes undocumented.
Logging medical device information separately for each case also reduces paperwork and data entry errors. Casework can be archived and accessed using keywords.
3. Support for Medical Device Billing
Billing is a critical part of medical device management with virtually no margin of error. A system that supports automation will thus help tremendously in reducing data entry errors and other human errors.
On the other hand, an ERP system for medical devices should also have a Purchase feature that can be used to generate purchase orders and be shared automatically with the billing team based on inventory levels.
4. HIPAA Compliant
Any software that has access to medical must be HIPAA-compliant, your ERP system is no exception. Every part of the ERP system needs to meet the requirements outlined in the HIPAA law. Data transmission needs to be fully encrypted at all times and backed up to secure cloud servers daily.
Additionally, role-based access control will enable granular user access control and ensure sensitive data is only accessed by authorized personnel.
5. Data Consistency and Integration
There should be no discrepancy in the data stored by medical device manufacturers. To avoid discrepancies and ensure complete transparency, the data collected and stored in an ERP system should be safely and easily shared between both parties. Ideally, the ERP system should support native integration to EMR systems used by manufacturers to avoid data redundancy and errors.
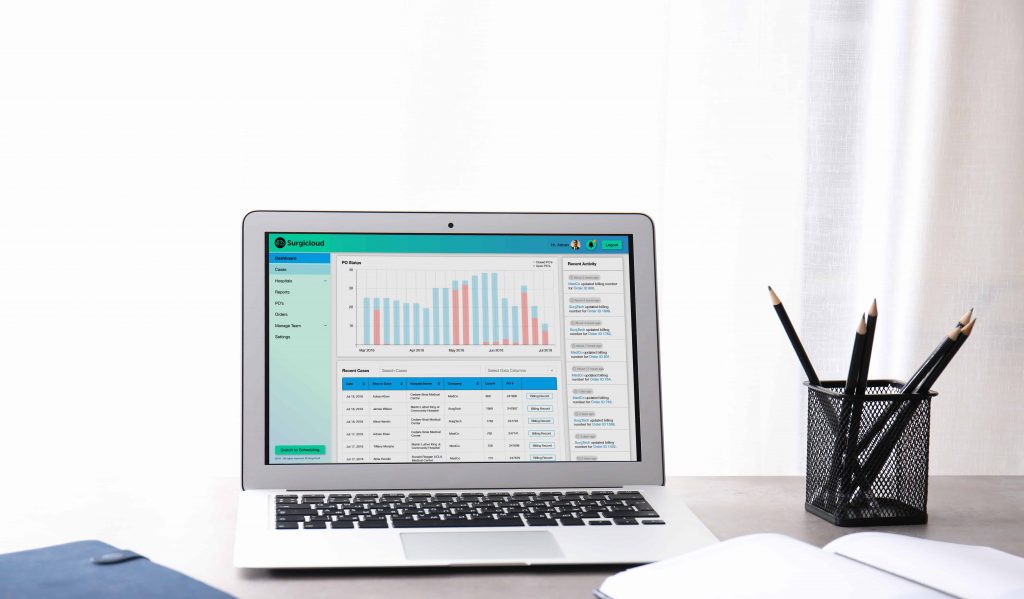
How Surgicloud Helps
There is no getting around it, medical device manufacturers who leverage data-driven solutions maintain a larger competitive advantage and thus, a larger market profit share.
As continued technological developments introduce more advanced medical devices, there is increased global demand for supply chains besides stricter compliance standards. The only way for any manufacturer to stay ahead of the ever-growing demands is via the right ERP solution. But what is the right ERP solution?
Surgicloud is a cloud-based inventory management, billing, and scheduling solution that optimizes and coordinates and device billing for hospitals’ surgical teams, medical device manufacturers, and distributors. It also meets all of the requirements of an ideal ERP system discussed in this article.